What is gamma scintigraphy?
It’s a non-invasive imaging technique which was first used in the medical industry before transitioning into clinical development. The basic principle involves incorporating a low level of gamma emitting radionuclide (which emits energy in the form of gamma rays) into a drug product or foods and fluids which are then administered to healthy volunteers or patients. A gamma camera is then used to detect the gamma rays being emitted, providing detailed information on the deposition, dispersion and movement of the radionuclide within the body. The technique allows visualisation and quantification of the dosed radioactivity, to support the understanding of what happens to a drug product following administration.
Quotient has extensive expertise in the delivery of scintigraphic studies, along with the facilities and processes required, including our three state of the art gamma cameras.
Figure 1: One of Quotient’s gamma cameras used for scintigraphy studies.
Scinti one
Figure 2: Schematic of gamma camera and data collection used in scintigraphy studies.
Scinti 2
How is gamma scintigraphy used by development teams?
Scintigraphic studies can be a useful tool in early development, as well as generating data to support marketing claims for products already on the market, or close to being approved. Quotient uses scintigraphy to investigate the performance of oral, inhaled and rectal drug products. These studies are used to:
Provide proof of concept for novel formulationsAssist with formulation, technology or device selection and optimizationCompare against competitor productsAssist with problem solving when visual data is required to assess the full pictureEvaluate pharmacodynamic responses such as mucociliary clearance mechanisms or GI (gastrointestinal) motilityFor oral products, some of the key questions that can be addressed are:How long does it take for a dosage form to transit through regions of the gastrointestinal tract?How long does it take drug release to occur from an oral formulation and where in the gastrointestinal tract has this occurred?What effect does food and gastrointestinal pH have on an orally administered formulation?Does the formulation behave in vivo as predicted in vitro
Figure 3: Representative scintigraphic images from typical oral drug delivery studies
Scinti 3a.
Assessment of oesophageal transit of an orally administered suspension formulation (Far left: radioactivity present in the mouth, to far right: all radioactivity present in the stomach)
Scinti 4
b. Assessment of gastrointestinal transit of a discrete formulation to assess the time and location of drug release following oral administration
For inhaled products, some of the key questions that can be addressed are:
- Which part of the lungs does the drug reach, and at what quantity?
- How does subject positioning and inhalation technique affect drug delivery to the target region?
- Does the location of drug deposition from an inhaled device change over time?
- Does the administration of the drug improve clearance of mucus from the lungs?
- Has targeted nasal delivery been successful for the administration of systemically acting drugs? (And has any drug reached the sinus or olfactory regions?)
- Can pulmonary deposition be achieved from a nasally administered product?
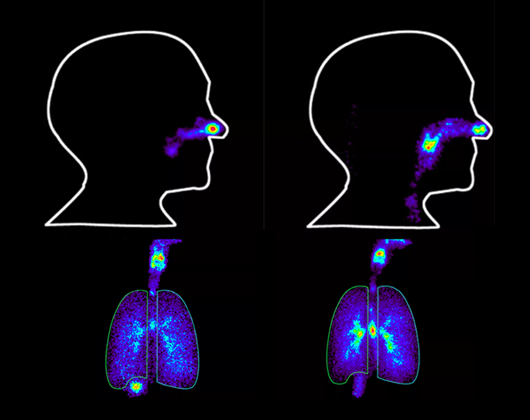
Figure 4: Representative scintigraphic images from typical inhalation studies
scinti
What data and images are collected and why?
Gamma cameras help development teams to visualise the gamma emitting radionuclide while within the human subject. Following dosing of the drug l product to the human subject, regular images are taken using the gamma cameras. The timing and duration of the images are determined on a case-by-case basis for each study, to take into consideration the study design, objectives and desired outcomes. For example, if the drug product is a fast acting or immediate release dosage form, there will be a high frequency of images taken of the subject for the first 1-2 hours following dosing. Whereas for modified release dosage forms which are intended to release drug over long periods of time, images could be collected over an 6-12 hour period.
Where needed, scintigraphic assessments can be performed in combination with pharmacokinetic (PK) analysis to generate an enhanced understanding of product performance; this is termed ‘Pharmacoscintigraphy’. This can prove useful in instances where interpretation of the PK data alone can present challenges, or when it is anticipated that the transit and disintegration of an oral formulation may significantly affect the PK data. Locally acting drug products can particularly benefit from this type of analysis, where performance of the dosage form cannot always be assessed accurately by standard PK approaches alone, as the drug concentrations in systemic circulation may not be easily measurable.
Learn more about Quotient Sciences scintigraphy capabilities