Selecting the right outsourcing partner for your drug development program is an extremely complex process. Within the pharmaceutical industry today, drug developers are increasingly outsourcing pre-clinical development, clinical research, and drug product manufacturing to external contract service providers (contract development and manufacturing organizations [CDMO] and contract research organizations [CRO]). While this can provide benefits, such as the ability to leverage external expertise and technologies, it can be challenging for the drug developer to manage multiple vendors, projects can be slow to start, disconnects can lead to delayed timelines, and it can be extremely costly. This points to a need for a simplified outsourcing model.
How does an integrated services model accelerate drug development?
Traditionally, the pharmaceutical industry has been structured around functional silos. In a conventional outsourcing approach, a sponsor has to split their drug development program between one or more CDMOs and a separate CRO. This places the project management burden on the sponsor, creates gaps in the development timeline, and limits knowledge and material sharing. Ultimately, this restricts productivity, slows down the drug development process, and is costly for the sponsor.
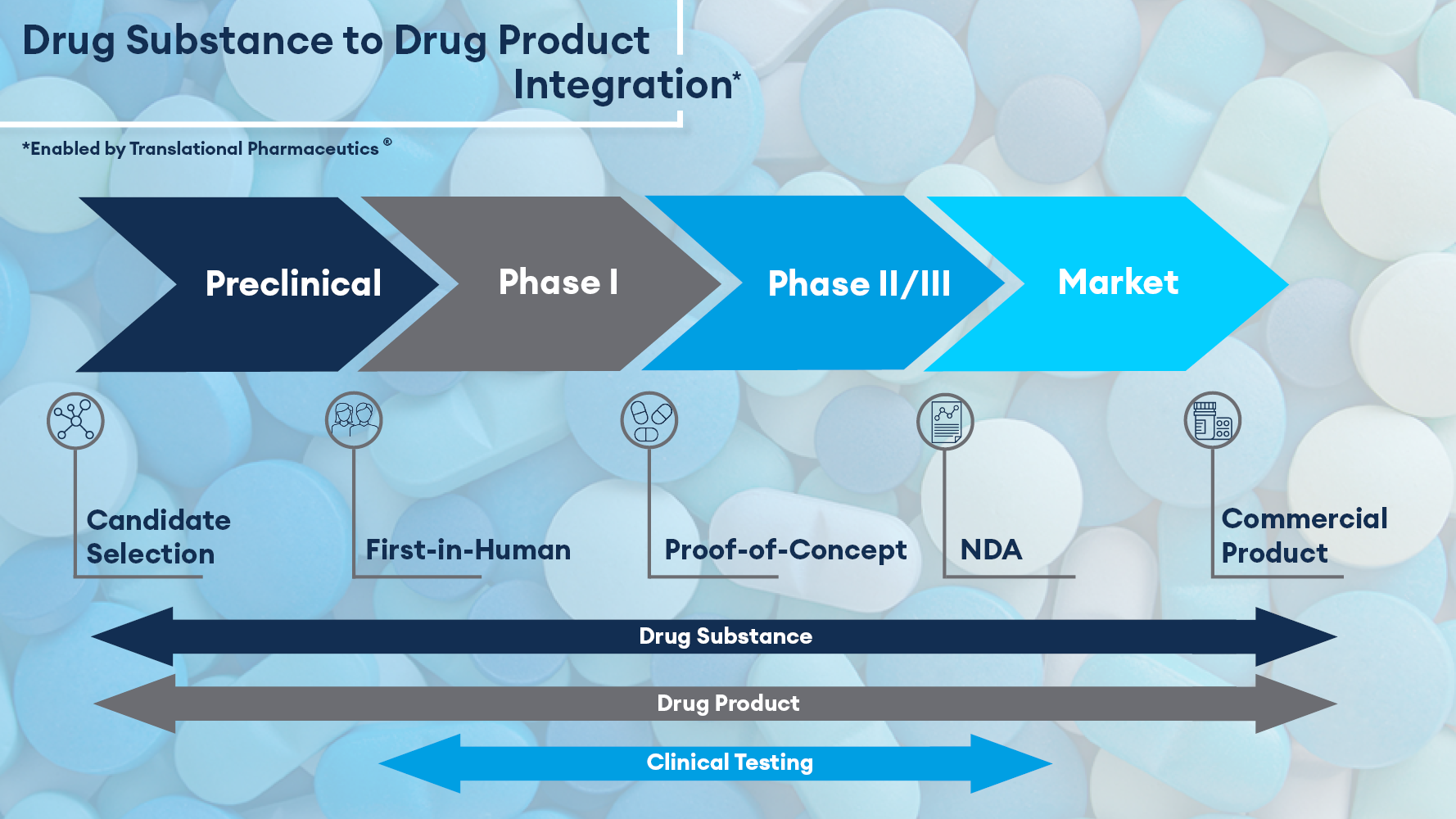
At Quotient Sciences, we have the unique ability to integrate drug substance, drug product, and clinical testing activities all under one organization and a single program manager. This unique platform is called Translational Pharmaceutics®, and it breaks down traditional industry silos to accelerate molecules through development. This streamlined approach seamlessly supports our customers’ programs across the entire drug development pathway, from candidate selection through to commercialization.
How does Quotient Sciences’ unique Translational Pharmaceutics platform work?
Translational Pharmaceutics employs rapid ‘make-test’ cycles, where drug products are manufactured, released, and dosed in a clinical study in days rather than months, shortening the time to decision-making human data. Emerging clinical results inform formulation composition selections in real-time, maximizing the potential for success by avoiding the risk of relying on up-front, non-predictive surrogate tools.
We have also integrated drug substance activities into our platform, further streamlining early development activities. Precious active pharmaceutical ingredient (API) material can be conserved and critical path activities can be minimized when supplying materials for Good Laboratory Practice (GLP) toxicology and first-in-human (FIH) clinical studies.
Our expertise in understanding the scientific dependencies between drug substance properties, formulation design, and clinical outcomes therefore enables us to enhance development efficiency. By closely aligning drug substance, drug product manufacturing, and clinical testing workflows, this encourages close relationships between multidisciplinary experts and creates a more agile approach to pharmaceutical development. The addition of drug substance into our integrated platform enables us to shorten the time from candidate development to FIH, accelerate molecules from FIH to proof of concept (POC), select and optimize clinical formulations, and accelerate products to commercial manufacturing.
What sets Quotient Sciences apart from other contract service providers?
Many contract service providers claim that they provide fully integrated solutions, but in reality, that is not the case. Quotient Sciences is currently the only outsourcing partner able to offer sponsors the ability to manufacture, release, and dose under one organization.
Translational Pharmaceutics has a long-standing history and proven track record of delivering integrated programs in the US and the UK. The unique platform is approved by the UK Medicines and Healthcare products Regulatory Agency (MHRA) and the US Food and Drug Administration (FDA), and it has been used by pharmaceutical and biotechnology companies on over 500 drug programs. We also have over 200 scientific publications on the topic in the public domain.
Why does proven, in-house expertise really matter?
As an organization, Quotient Sciences has over 30 years of experience in drug substance, drug product development, and clinical testing. This year, we celebrate 15 years of Translational Pharmaceutics and the design and delivery of integrated programs based on the unique needs of our customers’ molecules. Our highly skilled scientists and cross-functional teams work closely together and share knowledge and materials for the duration of a project.
Each cross-functional project team is overseen by a single program manager, who seamlessly manages the end-to-end process, provides regular status updates, and ensures timely project delivery for our customers. We work with clients of all sizes, from small biotechnology companies to large pharmaceutical companies, tailoring our services to meet their specific project goals.
What are the different applications of Translational Pharmaceutics?
Translational Pharmaceutics has been used by customers across the whole of the development life cycle, to develop new chemical entities and manage the life cycle of existing commercial products.
Translational Pharmaceutics can assist at each development phase, including:
- closely aligning workflows around drug substance synthesis, formulation development, drug product manufacturing, and clinical testing to shorten the time from candidate development to FIH
- accelerating molecules from FIH to POC in patients
- selecting and optimizing clinical formulations based on human data
- accelerating products to commercial manufacturing and launch.
While the majority of studies have been performed for oral molecules, the platform has been used for all routes of delivery, including:
- parenteral (intravenous, subcutaneous, and intramuscular)
- inhaled (pulmonary and nasal)
- topical
- ocular.
For complex molecules, key technologies for oral delivery that can been applied include:
- solubility and bioavailability enhancement (for example, via particle size reduction, amorphous dispersions, and lipidic systems)
- modified-release formulations (for example, matrix tablets, coated tablets/multiparticulates for sustained/delayed release, and gastroretentive technologies)
- taste-masking strategies for pediatric dosage forms.
What are the overall time and cost-saving benefits of Translational Pharmaceutics?
In 2020, Quotient Sciences commissioned the Tufts Center for the Study of Drug Development (CSDD) to conduct a study comparing completed Translational Pharmaceutics programs to benchmarked industry drug development timelines. The study concluded that for programs utilizing Translational Pharmaceutics, on average, development timelines were reduced by over 12 months, delivering financial gains of more than $200 million per approved new drug through a combination of reduced R&D costs and earlier access to commercial sales.
What is next for Translational Pharmaceutics?
In February 2022, a year after the acquisition of our Alnwick, UK, site, Quotient Sciences announced the integration of drug substance into the Translational Pharmaceutics platform.
By having both drug substance and drug product manufacturing activities under one organization, we can now deliver integrated chemistry, manufacturing, and controls (CMC) development activities for both pre-clinical and clinical studies in parallel, simplifying the supply chain and shortening the time from candidate selection to clinic by a further 3–6 months.
As we look to the future, Quotient Sciences will continue to bring on new services that further integrate drug development and streamline the outsourcing needs of our customers.
For more information about Quotient Sciences’ fully integrated Translational Pharmaceutics platform, click here.